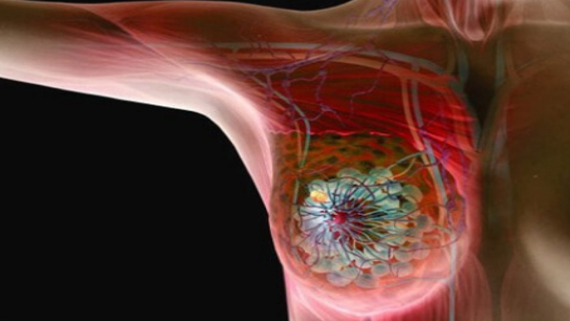
Results were released this week on a new treatment with the potential to improve the outcome for patients with hereditary BRCA mutations and high-risk, early-stage breast cancer. These results represent the first time a drug that blocks cancer cells from repairing their DNA (called a PARP inhibitor) has been shown to significantly reduced the risk of breast cancer returning in high-risk patients following completion of standard Chemotherapy, Surgery and Radiation therapy.
Titled “ Adjuvant Olaparib for patients with BRCA1 or BRCA2 Mutated Breast Cancer,” The paper appears in the New England Journal of Medicine and was presented June 6 as the first abstract during the plenary session at the 2021 American Association of Clinical Oncology (ASCO) annual meeting. Lead author and the OlympiA trial Steering Committee Chair Andrew Tutl, M.D, Ph.D, of the Institute of Cancer Research and King’s College London was principal investigator on the portion of study conducted in patients outside the U.S and present the results at ASCO.
Led by top experts in BRCA- associated breast cancer from around the world, the OlympiA trial’s co-chair were Charles E. Geyer, Jr, M.D a breast medical oncologist and deputy director of the Houston Methodist Cancer Center Judy E. Gather, M.D , M.P.H, of the Dana- Farber Cancer Institute and Bella Kaufinan M.D of the Sheba Medical Center in Israel. Geyer was the principal investigator on the NCI- sponsored portion of the study conducted in the U.S.
“OlympiA represents a successful global collaboration among leading international academic breast cancer research groups. Cancer genetics experts, the National cancer institute and pharmaceutical industry partners to evaluate the efficacy and safety of Olaparib to address the unmet need for improved therapy for an individual with high-risk BRCA mutations- associated early breast cancer”, said Geyer who is a professor of Medicine Research Institute and has provided scientific leadership on the trail since 2013.
Read also: Tuberculosis: New biomarker indicates individual treatment duration
The OlympiA trial was a tremendous effort recruiting 1836 patients from 420 centres across 23 countries. A randomized double-blind phase 3 trial, OlympiA was designed to test the efficacy of the poly (ADP- ribose)-polymerase (PARP) inhibitor drug Olaparib and showed that it significantly improved invasive and distant disease-free survival when given for 52 weeks following the completion of such standard therapies as Chemotherapy, Surgery and Radiation.
Patients were recruited from June 2014 to May 2019 and patients who consented to participate were randomly assigned to receive Olaparib or a placebo. After three years following initiation of treatment with Olaparib 85.9 percent of patients were alive and free of recurrent invasive breast cancer and new second cancer, compared with 77.1 percent of patients who received a placebo. During this same time frame, 87.5 percent of patients receiving Olaparib were alive and free of distant metastatic disease, compared to 80.4 percent on the placebo.
While Olaparib was also associated with 27 fewer deaths than those on placebo, researchers say longer blinded follow up is required to assess the impact of this therapy on overall survival. What is certain, says the researchers, is that germline BRCA1 and BRCA 2 sequencing is becoming an important biomarker for the selection of systemic therapy in early breast cancer.
By: Peace Chigozie